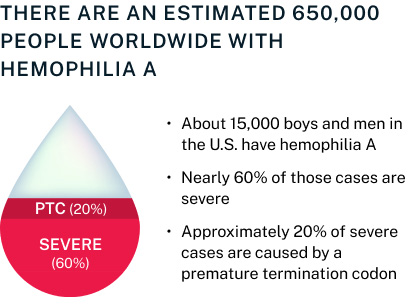
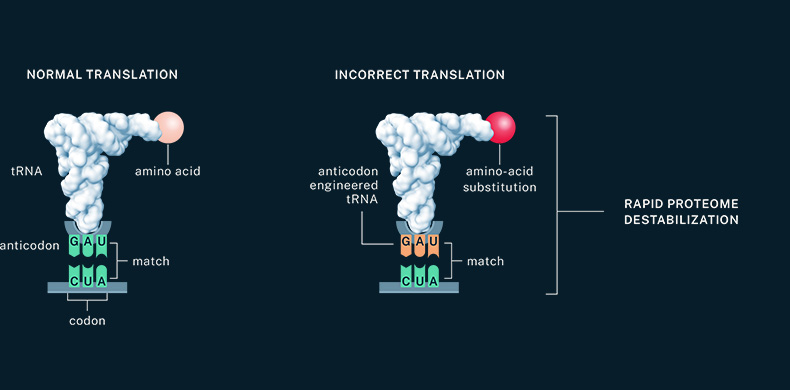
Our lead program in severe hemophilia A is supported by preclinical data that includes in vitro assays demonstrating tRNA-mediated restoration of full-length clotting protein FVIII and that specifically shows activity in cells containing a PTC in the protein’s open reading frame.
We are building on these data through IND-enabling studies with the goal of enrolling a phase 1 clinical trial. Because of the versatility of tRNA-based therapies, approval in severe hemophilia A can act as a proof of concept and unlock treatments for a range of bleeding disorders.